Patients and Caregivers
Worried about severe low blood sugar?
BAQSIMI is here to help
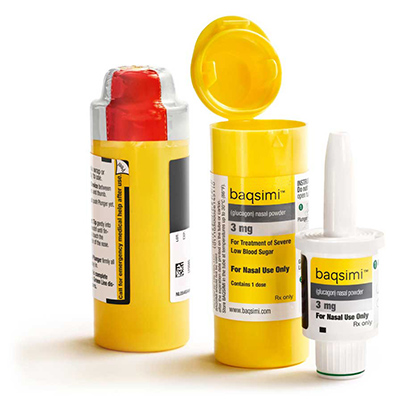
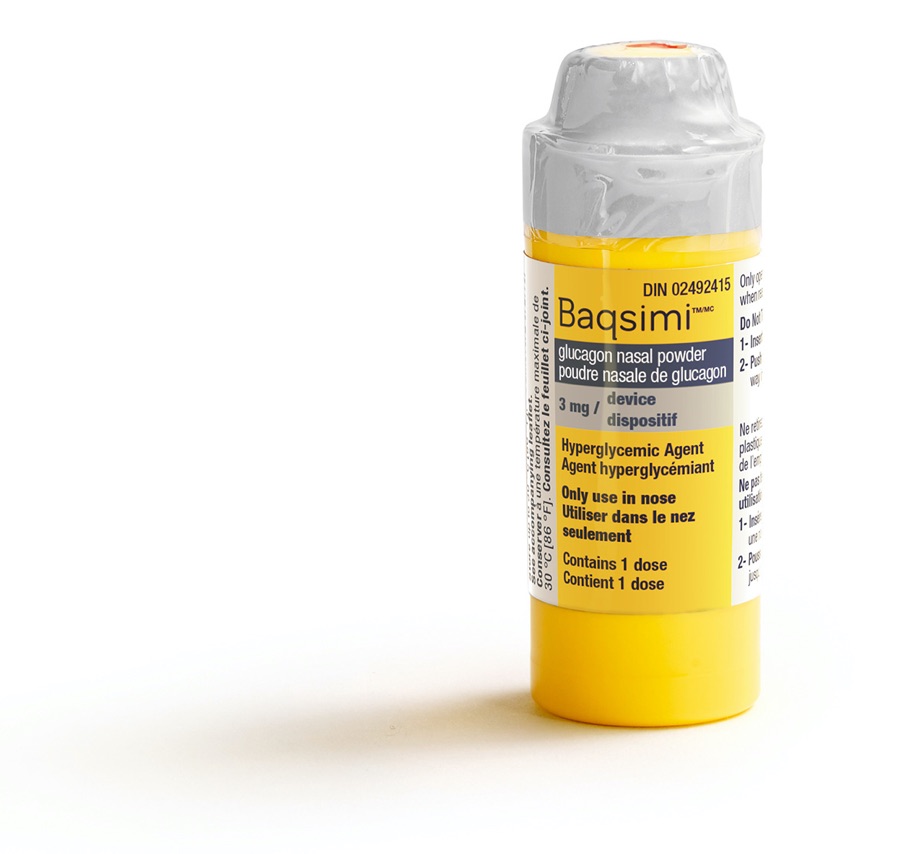
BAQSIMI® is a medicine used to treat severe low blood sugar. It’s given to someone during a low blood sugar emergency (severe hypoglycemia) when they are unable to eat, drink, or swallow sugar on their own, and it’s meant for adults and children 4 years and above who take insulin for diabetes.
Answer these simple questions to find out if BAQSIMI is right for you:
1. Do you have diabetes and take insulin?
2. Have you ever had any of these low blood sugar symptoms?
3. Have you ever needed another person’s help during a low blood sugar emergency?
4. Have you ever experienced a time when you were unwilling or unable to consume oral carbs?
5. Have you ever had a blood sugar level below 2.8 mmol/L?
6. Do you ever worry or feel anxious about experiencing a low blood sugar emergency?
If you answered ‘yes’ to any of the questions above, ask your doctor about a rescue plan that includes BAQSIMI.
You may be at risk of low blood sugar emergencies and might have already experienced one in the past if you answered ‘yes’ to any of the questions above. To help you bring this up with your doctor, download and share the following guide.