How to use BAQSIMI
How to use BAQSIMI?
Show your family, friends, or coworkers where you keep BAQSIMI and explain how to use it by sharing these instructions.
They need to know how to use BAQSIMI before you need it.
Important
- Do not remove the shrink wrap or open the tube until ready to use
- Do not test the device before use, as it contains only one dose of glucagon and can only be used once
- Only use BAQSIMI in the nose
- Read the BAQSIMI Patient Medication Information found inside the product box before using
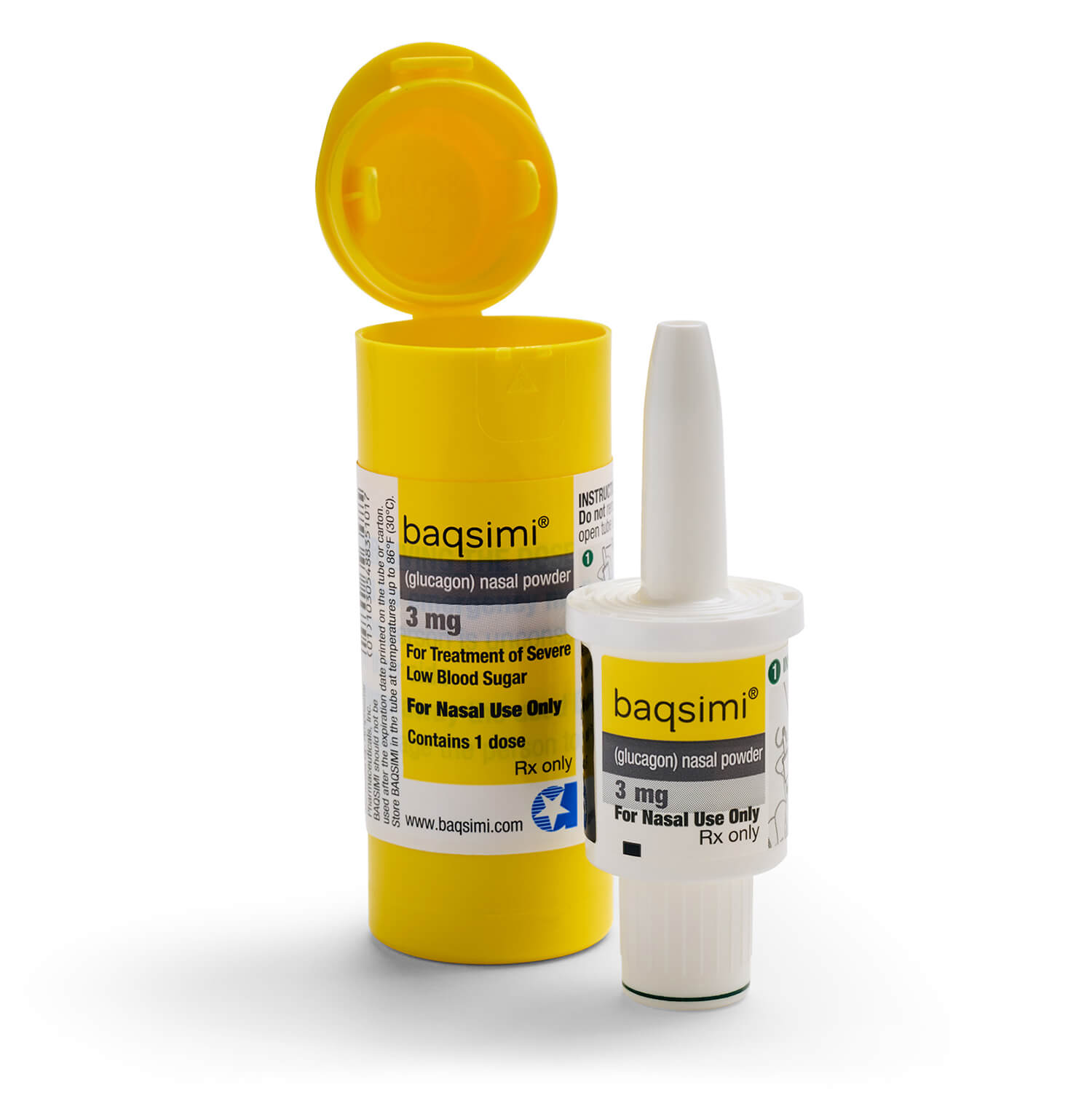
Preparing the dose:

Remove the Shrink Wrap by pulling on the red stripe.

Open the Lid and remove the Device from the Tube.
Caution: Do not press the Plunger until ready to give the dose.
Giving the dose

Hold Device between fingers and thumb.
Do not push Plunger yet.

Insert Tip gently in one nostril until finger(s) touch the outside of the nose.

Push Plunger firmly all the way in. Dose is complete when the Green Line disappears.
Watch the following video to learn how to administer BAQSIMI or read the steps below. Share it with your family, friends or coworkers so they can administer BAQSIMI in case of emergency.

